 |
 |
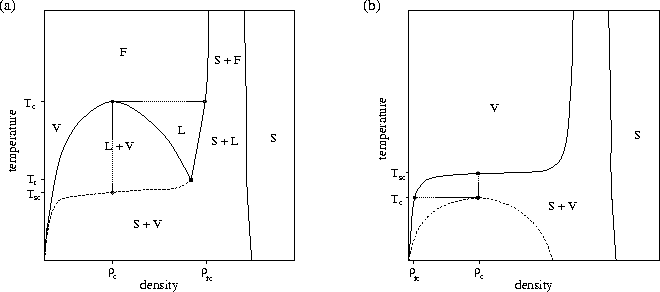
In contrast to C60, for which the intermolecular potential is very short-ranged with respect to the equilibrium pair separation, the critical temperature for sodium is about seven times larger than the triple point temperature because of the long-ranged interatomic forces. The results for C60 have led to a flurry of studies examining the effect of the range of the potential on the phase diagram[182,183,184,185]. These investigations have clearly shown that as the range of attraction decreases, the difference between the triple and critical temperatures decreases until the critical temperature drops below the triple point and the liquid phase disappears. Similar effects have previously been noted for mixtures of spherical colloidal particles and non-adsorbing polymer by theory[120], simulation[186] and experiment[121,122,123]. For such systems, the size of the polymer can be used to vary systematically the range of attraction between the colloidal particles.
Although the phenomenology of the range-dependence of the liquid phase stability is clear, a structural explanation has not been given. In this chapter we provide such a microscopic view by relating the above effects to fundamental changes in the topography of the PES (§4.3) and by making a detailed connection between these changes and liquid structure (§4.4). By studying both clusters and bulk we can address questions concerning the emergence of the phase-like forms of clusters and their evolution to the bulk limit. Detailed simulations of the thermodynamic properties of a 55-atom cluster (§4.5) confirm that our results can explain the range-dependence of the thermodynamics. We can also suggest an explanation for the transition from electronic to geometric magic numbers observed in the mass spectra of sodium clusters[53] (§4.6), showing that the simple approach we describe here can provide insight into a diverse set of phenomena.