 |
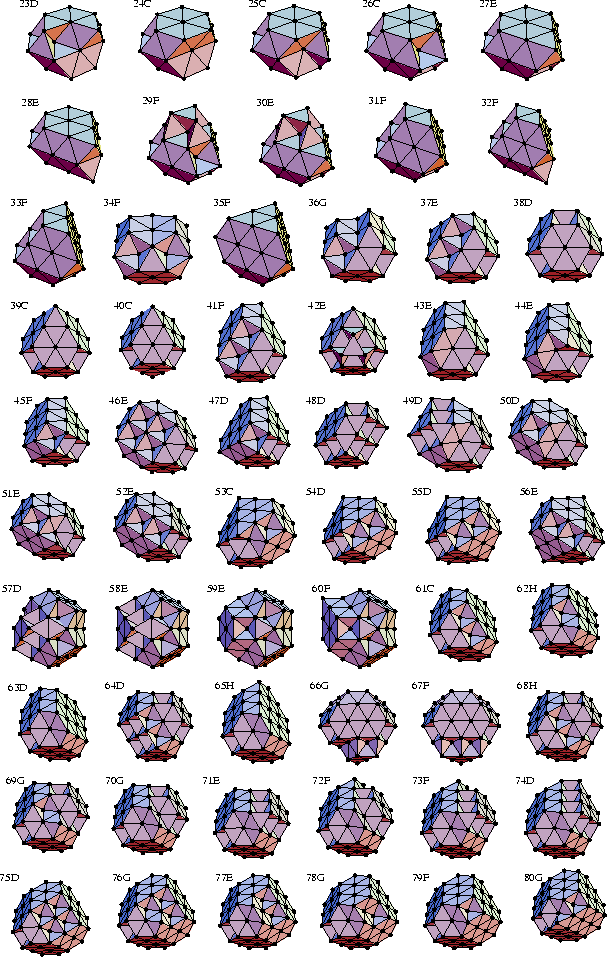
The 38-atom truncated octahedron, 38D, is the most stable fcc cluster in the size range we consider here.
It becomes the global minimum at the lowest value of ![]() (4.76) of any of the close-packed structures.
Curiously, there are two ranges of
(4.76) of any of the close-packed structures.
Curiously, there are two ranges of ![]() for which it is the global minimum.
At long range, Ennn represents a significant part of the total energy.
The truncated octahedron is most stable for
for which it is the global minimum.
At long range, Ennn represents a significant part of the total energy.
The truncated octahedron is most stable for ![]() because it is approximately spherical
and so has a larger value of Ennn than the more oblate icosahedral structure 38E.
For shorter-ranged potentials, the contribution of Ennn diminishes and so 38E becomes
the global minimum for
because it is approximately spherical
and so has a larger value of Ennn than the more oblate icosahedral structure 38E.
For shorter-ranged potentials, the contribution of Ennn diminishes and so 38E becomes
the global minimum for ![]() because it has a larger nnn.
Then for
because it has a larger nnn.
Then for ![]() the truncated octahedron again becomes the global minimum because it has
a lower strain energy than 38E.
There is a growing body of experimental evidence for the importance of truncated octahedra.
Parks et al. have recently assigned this structure to Ni38 by probing the cluster's
chemical reactivity.[11]
EXAFS (extended x-ray absorption fine structure) spectra of small gold clusters
have been interpreted in terms of the presence of truncated octahedral clusters,
particularly the 38-atom truncated octahedron.[78]
Gold clusters passivated by alkylthiolate molecules selectively form truncated
octahedra, which can be isolated and formed into superlattices.[31,32]
This structure is also observed for ligated 38-atom platinum clusters.[79]
the truncated octahedron again becomes the global minimum because it has
a lower strain energy than 38E.
There is a growing body of experimental evidence for the importance of truncated octahedra.
Parks et al. have recently assigned this structure to Ni38 by probing the cluster's
chemical reactivity.[11]
EXAFS (extended x-ray absorption fine structure) spectra of small gold clusters
have been interpreted in terms of the presence of truncated octahedral clusters,
particularly the 38-atom truncated octahedron.[78]
Gold clusters passivated by alkylthiolate molecules selectively form truncated
octahedra, which can be isolated and formed into superlattices.[31,32]
This structure is also observed for ligated 38-atom platinum clusters.[79]
Structures 50D and 79F both have D3h symmetry and a single twin plane passing
through the structure.
The two halves of the structure have the surface morphology of truncated octahedra.
Indeed 79F can be formed from the 79-atom truncated octahedron by rotation of one half of the structure
by 60![]() about an axis perpendicular to one of the
about an axis perpendicular to one of the ![]() planes.
Both structures have the same number of nearest neighbours and 79F is slightly lower in
energy only because of the larger contribution from next-nearest neighbours that
results from the twin plane.
Again there is recent experimental evidence for the stability of this type of structure
for gold clusters,[34] although at larger sizes (N=225 and 459)
than considered in this study.
planes.
Both structures have the same number of nearest neighbours and 79F is slightly lower in
energy only because of the larger contribution from next-nearest neighbours that
results from the twin plane.
Again there is recent experimental evidence for the stability of this type of structure
for gold clusters,[34] although at larger sizes (N=225 and 459)
than considered in this study.
The closed-packed structures from N=57-60 are based on 59E.
This structure is a 31-atom truncated tetrahedron with the faces covered by four seven-atom hexagonal
overlayers occupying hcp sites with respect to the underlying tetrahedron.
The stability of 59E comes from the combination of its high proportion of ![]() faces
and its spherical shape.
If atoms are added to one of the grooves in 59E a decahedral-like
axis results, indicating a possible path between decahedral and closed-packed clusters.
faces
and its spherical shape.
If atoms are added to one of the grooves in 59E a decahedral-like
axis results, indicating a possible path between decahedral and closed-packed clusters.
It is worth noting that the cuboctahedron is not the lowest energy close-packed structure for N=55. In fact, it has four fewer nearest neighbours than structure 55D. Hence, when magic numbers occur at sizes corresponding to both complete Mackay icosahedra and cuboctahedra[25,26] (N=13, 55, 147, ...) it is more likely that they are due to icosahedra. Furthermore, one has to interpret with caution those studies which seek to find the relative stability of fcc and icosahedral structures by comparing cuboctahedra with Mackay icosahedra[80,81,82,83] because the cuboctahedra are likely to be suboptimal fcc structures.
Although this conclusion does not simply carry over to clusters surrounded by ligand shells--the
ligands could significantly modify the relative surface energies of ![]() and
and ![]() faces--it
is interesting to note that a recent reinvestigation of clusters which were originally thought to be
cuboctahedral 55-atom gold clusters[84,85]
seems to disprove this structural assignment.[86]
faces--it
is interesting to note that a recent reinvestigation of clusters which were originally thought to be
cuboctahedral 55-atom gold clusters[84,85]
seems to disprove this structural assignment.[86]