


Next: The slope of the
Up: Modified Potential Energy Surfaces
Previous: Modified Potential Energy Surfaces
Home: Return to my homepage
The first modification that we make is to change the barrier height.
Unsurprisingly, increasing b leads to a reduction in the rate of relaxation to
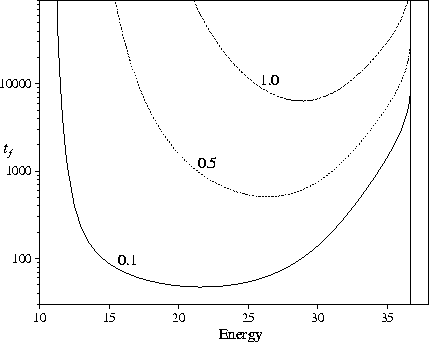
the global minimum (Figure 5.6).
More interesting is the dependence of this effect upon the energy:
increasing the barrier heights leads to a much larger increase in tf at low energies.
This is because a larger fraction of the kinetic energy must be converted to potential energy
to overcome the barriers at low energies.
The energy at which the maximum folding' rate occurs increases as the
barrier heights increase. The shape of the minimum in tf is also dependent
on b, becoming broader as the barrier heights are decreased;
at b=0.01 there is a larger energy window for which fast relaxation to
the global minimum can occur.
Figure 5.6:
The dependence of tf on the barrier height, b, for our standard PES.
tf is defined by P1(tf)=0.8.
The vertical dotted line denotes the energy at which P1eq=0.8.
Each line is marked by the value of b.
 |
Similar behaviour is observed for the canonical ensemble, namely
the rate of relaxation to the global minimum has a 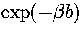 dependence on the barrier height.
Therefore, at fixed temperature the relaxation rate has a rigorously exponential dependence on b,
and changing b has a greater effect on the relaxation rate at low temperature.
dependence on the barrier height.
Therefore, at fixed temperature the relaxation rate has a rigorously exponential dependence on b,
and changing b has a greater effect on the relaxation rate at low temperature.
These results can easily be understood in the language of
Bryngelson and Wolynes[243,244,245]:
increasing the barrier heights corresponds to increasing Tg without changing Tf,
hence decreasing the ratio Tf/Tg and the folding' ability.
Potassium chloride clusters provide a good example of systems for which relatively small barrier heights
produce rapid relaxation down the PES[252].



Next: The slope of the
Up: Modified Potential Energy Surfaces
Previous: Modified Potential Energy Surfaces
Jon Doye
8/27/1997


![]() dependence on the barrier height.
Therefore, at fixed temperature the relaxation rate has a rigorously exponential dependence on b,
and changing b has a greater effect on the relaxation rate at low temperature.
dependence on the barrier height.
Therefore, at fixed temperature the relaxation rate has a rigorously exponential dependence on b,
and changing b has a greater effect on the relaxation rate at low temperature.